Good Medicine, Bad Behavior: Drug Diversion in America
Explore the Exhibit
The History of Prescription Drugs
- 1890s - 1940s
- 1950s - 1970s
- 1980s - Present

1900 - Opium, morphine and cocaine in many patent medicines leads to addiction and death. Mrs. Winslow’s Soothing Syrup kills many children each year due to overdosing on morphine. Morphine is the syrup’s primary ingredient but it is not listed on the label.
A Turn-of-the-Century Pharmacy (1890s-1940s)
During the past 120 years there has been a revolution in therapeutics. Medicines have been discovered to cure disease and to relieve pain. With these new medicines there is an increase in the misuse and abuse of some of them. This unit will look at some of these medicines and the abuses and the laws that have developed to help control the misuse of those medicines. It is important to keep in mind that prescription medicines can be just as dangerous when misused as street drugs.
1898 - Heroin is introduced. First thought to be non-addicting, but founded to be so addicting that health professionals, legislatures and the public call for its ban or control.
1899 - Aspirin introduced into medical practice due to its effectiveness, Bayer Company of Germany originally promotes it to doctors. This is the first safe and effective medicine that is not addictive.
1900 - Opium, morphine, heroin and cocaine in wide use in over-the-counter medicines made by a pharmacist or a manufacturer (known as patent medicines).
1903 - Barbiturates introduced as hypnotic (sedative), soon replace the more toxic bromides that are commonly used for headaches and stress. Bromides are in popular products like Bromoseltzer. People who become addicted to bromides might consume 3 to 5 glasses a day or more. Bromides are discontinued in products soon after World War II.
1912 - Hague Opium Convention Treaty in China. The United States signs international agreement to limit the spread and use of narcotics.
1914 - Harrison Act uses IRS taxes on the sale and purchasing of narcotics as a way to control use. The Treasury Department assigns the first narcotic agents to enforce the Harrison Act.
1919 - United States v. Doremus court case confirms that the federal government can regulate dispensing of medicines by physicians.
1919 - Webb et al v. United States court case confirms that physicians and pharmacists cannot supply an addict just to maintain his or her addiction.
1920s - Enforcement of the Harrison Act and the resulting restricted access to narcotics starts the smuggling of drugs from abroad. In this image, agents pour out liquid used to cover sealed packages of narcotics.
1928 - The Prohibition of alcohol. Doctors can write special prescription for pints of whiskey or wine for their patients. Millions of prescriptions are written and dispensed. Much of this alcohol is not used for medical purposes, resulting in the greatest diversion of prescription medicines prior to the present time.
1933 - Repeal of Prohibition. Organized crime groups who are put out of the liquor business turn to smuggling and selling narcotics.
1930 - Federal Bureau of Narcotics was formed. Harry J. Anslinger leads the bureau until 1962. Anslinger supports drug addiction research. A Public Health Service Hospital is built in Lexington, Kentucky. It is the United States government’s center for narcotic research and addiction treatment.
1930s - Law enforcement leads to diminished abuse of cocaine, opiates and marijuana.
1933 - Introduction of amphetamines into the United States. The military on both sides of WW II uses amphetamines. It is given on both sides to assist soldiers in staying awake during days of combat. Amphetamine use by civilians does not increase until after the war.
1937 - Marijuana Tax Act. Marijuana comes under federal control.
1938 - Amendments to the Pure Food and Drug Act brings the abuse of non-narcotic medicines under the responsibility of the FDA.
Introduction

Explore the rise of patent medicine industry from the late 1800s through to modern day prescription drugs.
Learn More
Learn More
History of Prescription Drugs
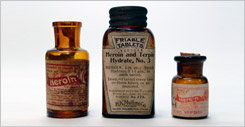
Explore the revolution in therapeutics during the past 120 years. Medicines have been discovered to cure disease and to relieve pain.
Learn More
Learn More
The Science of Drugs

Explore the many substances found to have wonderful healing and pain-relieving properties, but also many that could cause harm if used incorrectly.
Learn More
Learn More
The Controlled Distribution System

Explore the various laws and regulations have been passed by the federal and state governments to regulate and control the manufacture, distribution and dispensing of medicines.
Learn More
Learn More
Prescription Fraud

Explore the laws put in place in response to growing abuse and addiction problems with controlled substances.
Learn More
Learn More
Diversion of Chemicals

Explores the clandestine production of drugs, which is dependent on the availability of chemicals necessary to produce the illicit drug activity.
Learn More
Learn More
Pain Management
Explores the critical balance between promoting pain relief and preventing the diversion and abuse of powerful prescription drugs.
Learn More
Learn More
Lost Talent
Discovery Corner