Good Medicine, Bad Behavior: Drug Diversion in America
Explore the Exhibit
The Science of Drugs
- Introduction
- Medicines and the Body
- Too Much?
- The Science
“In the past 30 years, advances in science have revolutionized our understanding of drug abuse and addiction. Drug addiction is a brain disease.”
Nora D. Volkow, M.D.
Director, National Institute on Drug Abuse
Director, National Institute on Drug Abuse
Medicines have been around for as long as mankind has been around to need them. While many substances are found to have wonderful healing and pain-relieving properties, there are also many that could cause harm if used incorrectly. But what is it that these products actually do to the body? What changes and what harm do they do?
To understand the body’s response to the abuse of medications, we must first take a look at the human brain, and how it reacts to both natural chemicals and to drugs and medicines, as well as how it communicates. When drugs and medicines interrupt the brain’s natural reward pathways, a cycle of addiction and abuse can be created and can have lethal consequences.
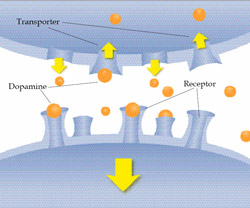

How drugs alter the brain's reward pathway.
The Pleasure Center of the Brain –
The Brain’s Reward Pathway
Brains are hard-wired to keep the species alive. Therefore, the necessary elements for doing this: eating, reproducing, communal living, etc., are meant to feel pleasurable so that they will be repeated and the species will continue. When these activities are performed, the brain rewards the body through a complex “pleasure center” which reinforces the desire to repeat the behavior. NIDA
How Do Neurons Communicate?
The cells of the brain communicate with each other in a manner similar to the way people communicate. To send a message, a brain cell releases a chemical (Neurotransmitter) into the space separating two cells called the synapse. The neurotransmitter crosses the synapse and attaches to proteins (receptors) on the receiving brain cell. This causes changes in the receiving brain cell and the message is delivered. NIDA, concept courtesy: B.K. Madras
How Drugs Alter the Brain’s Reward Pathway?
Drugs interfere with the body’s reward system by bypassing the natural course and going directly to the brain’s reward pathway. There, they mimic the effects of the brain’s neurotransmitters to falsely produce and prolong the pleasurable sensation and paving the way for addiction. Virtually, all drugs of abuse activate the brain’s dopamine system and the pleasure/reward pathway. The shape of the drug is similar to the dopamine receptor site and can “lock” into it, thus replicating many times over the effects of dopamine. Typically, dopamine increases in response to natural rewards such as food. When cocaine is taken, dopamine increases are exaggerated and communication is altered. NIDA
Molecular Model of Morphine
Morphine was first isolated in 1804 by Friedrich Sertürner and used for pain relief. Although very effective, it was extremely addictive. The base chemical structure of all opiates, (including morpine, heroin, codeine, oxycodone and hydrocodone) are identical. The addition of various molecules at strategic points attached to the original structure are what differentiate them.
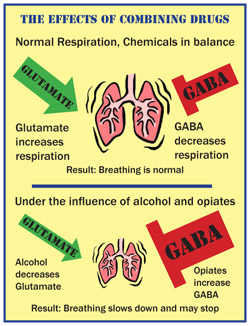
How Drugs Kill
With stimulants such as ephedrine (precursor chemical to methamphetamine) deaths usually occur as a result of brain damage, heart attacks, and/or overheating. Stimulants trigger the release of norepinephrine, a hormone which increases motor activity, heart rate and blood pressure, and also narrows blood vessels. The narrowing of blood vessels reduces the flow of blood around the brain and increases the risk of rupture which can lead to brain damage. As the heart pumps faster, it demands more oxygen and this, coupled with reduced blood supply, can lead to heart attack.
Stimulants also change the dopamine levels in the body, which effect body temperature, thus interfering with the body’s ability to cool itself. Along with the increased motor activity caused by the stimulant use, the body temperature rises to dangerous levels which can result in organ failure and ultimately death.
How Drug Combinations Can Affect Breathing
Many deaths occur as a result of combinations between drugs or medicines that, when taken together, can have lethal effects. Glutamate and GABA are two chemicals in the body that affect breathing. Glutamate is a chemical that works to increase breathing (excitatory). GABA is a chemical that works to decrease breathing (inhibitory). Normally these two chemical are in balance in the body. Alcohol decreases the effects of glutamate, causing breathing to slow down. Opiates(such as morphine, codeine and oxycodone) increase the effect of GABA, also causing breathing to slow down. When combined, the impulse to breathe can be totally suppressed, causing death.
Pharm Bowl
A growing trend among today’s youth is to attend Pharm Parties. Admittance to the party requires providing pharmaceuticals, usually illegally obtained from their parents’ home medicine cabinets. The pharmaceuticals are combined in a bowl or bag, and referred to as trail mix. Many times, handfuls of pills are washed down with alcohol, producing lethal drug combinations. Similar situations were found decades earlier when Manhattan socialites would provide Waterford candy dishes filled with Quaaludes pieces for the enjoyment of their guests.

Scientists at Brigham Young University researching to find a non-addicting potent pain reliever—Photo by Mark Philbrick.
Discovery and Development
The search for substances that can heal the body and relieve pain is never-ending. One substance ideal for pain relief – Opium – is also highly addictive. A major quest for scientists both past and present has been to find a non-addictive potent pain reliever. However, despite intensive and continued research, a non-addicting potent pain-killer has yet to be found.
Drug Development Process, from Discovery to Marketing
The process for developing a new medicine begins with the search for a compound, the subsequent testing, the manufacturing, and the final delivery of the product to the consumer. Drug development, from discovery to approval is a time consuming process. Total drug development time grew from an average of 8.1 years in the 1960s to 11.6 years in the 1970s, to 14.2 in the 1980s, to 15.3 years for drugs approved form 1990 through 1995. Source: PhRMA (Pharmaceutical Research and Manufacturers of America)
The Quest for Non-addicting Analgesics
The search for a potent pain-reliever that is not addictive was a continuous mission for Harry J. Anslinger, the first commissioner of the Federal Bureau of Narcotics. Library of Congress/Acme Newspictures
Early Research and Development
The Bayer Company in Germany tried unsuccessfully to find a non-addicting potent pain reliever. While experimenting with morphine, they produced an opiate compound that appeared to have the non-addictive qualities they desired. Bayer marketed this new compound as a cough syrup and pain reliever under the trade-name Heroin, only later discovering it to be much more addictive than morphine. Dr. Michael A. Bozarth, University of Buffalo
Opium and its Derivatives
Opium and its derivatives are the most potent pain-relieving compounds known, but unfortunately, they also have the highest abuse potential. Opium products include morphine, codeine, heroin, laudanum, paregoric, oxycodone, and a variety of others.
Research at BYU
Scientists continue today to search for a potent non-addicting pain reliever. At Brigham Young University, researchers are studying the Japanese tape vine in Australia with the hopes to synthesize a molecule similar to the chemical structure of morphine. Mark Philbrick, Brigham Young University
Sources
Medicines have been derived from a variety of sources including plants and animals. Researchers throughout millennium have collected herbs, leaves, bark, and flowers, as well as insects, and other animal products in the hopes of finding a treatment for some illness.
Plant Sources for Various Medications
Since ancient times, medicines have been derived from plants, and many of the important ones in use today, such as opium and quinine are plant-derived.
Scientist Collecting Specimens in a Rain Forest
Today, scientists continue to collect plant specimens from around the world in the hopes of isolating a new medicine. DEA Museum
Animal Sources for Medicines
Substances produced from animals include estrogen from horses (Premarin), insulin from cows and pigs, steroid hormones and vaccines. Anabolic steroids are man-made compounds related to male sex hormones and have been heavily abused by athletes looking to increase their body mass and enhance performance.
Clinical Trials
Once compounds are collected from various sources, they are tested to see if they can be useful for treatment. This process is continually refined, with testing on animals, computer simulations, and research on the best type of drug delivery system. After that, clinical trials are performed with human subjects. The clinical trial process follow strict protocols outlined by various Drug Regulatory Authorities around the world, such as the US Food and Drug Administration (FDA), the European Agency for the Evaluation of Medicinal Products, and the Japanese Ministry of Health, Labor and Welfare.
The Drug Discovery Process
In order for scientists to produce a medicine to counteract a disease, they first need to determine what is causing the disease. With knowledge of how the disease works, they can pinpoint the molecule, or target that is responsible for the disease. They then test different chemical or biological compounds on these targets to see if they have an affect and then isolate the compounds for further development and testing.
Animal Testing
Animals are used extensively in preclinical research testing in the laboratory. FDA History Office
Clinical Trials
Once a compound has gone through all the pre-clinical research activities and it has been optimized and improved, it is ready for clinical trials. Dept. of Health and Human Services
FDA’s Involvement in the Drug Process
One mission of the Food and Drug Administration is to assure the safety, efficacy, and security of drugs, as well as to speed innovations that make medicines more effective, safer, and more affordable. FDA scientists review the results of all the clinical tests to make a determination if the medicine is safe enough for approval. Once it is approved, it becomes available. After approval, companies continue to monitor it, and must submit periodic reports to FDA, which occasionally require them to make additional studies, based on additional data submitted. FDA History Office
Manufacturing
When medicines are manufactured, they have to be formulated to contain the correct dosage. Besides the actual medicine, formulation may also include other compounds that could be used to control the release of the medicine to the body, to assist with uptake into the body, extend the shelf life, mask the taste, etc.
How Medicines are Formulated: The Manufacturing Process
As the medicine goes through the manufacturing process, it is again subject to compliances with the drug regulatory authorities. The FDA uses the current Good Manufacturing Practice (GMP) guidelines. DEA
Good Manufacturing Practice
To ensure quality control and that Good Manufacturing Practice guidelines are being met, drug regulatory agencies are authorized to conduct inspections on pharmaceutical manufacturing plants. FDA History Office
Delivery Methods
Medicines can be delivered into the body via a wide range of delivery systems. Because of the addiction and abuse potential of opium and its derivatives, there has been recent research on developing abuse-resistant delivery systems. Some examples are encapsulated pellets, osmotic pumps, packaging, and transdermal patches. If the medications are used as directed, they are safe. However, if diverted for illegal use, the technology can be circumvented by savvy street users.
Medicine Delivery Methods
There are a variety of delivery methods for medications. Many tablets are coated to time the release of the drug upon entering the intestinal system, so that it will not be destroyed by the low pH of the body’s stomach. Other drugs (such as oxycodone) are prepared in such a way that as it makes its way through the digestive system, the medicine is slowly released over time, thus prolonging its release into the bloodstream, and reducing the frequency for taking the medication. Photos.com
DEA’s Involvement in the Process
While the majority of the development process is done through the FDA, The Drug Enforcement Administration gets involved in a variety of areas. One way is when a drug manufacturer changes the formulation of a product to make it less prone to abuse and then petitions for a hearing with DEA to have the product lowered on the schedule of controlled substances.
DEA’s Involvement in the Development of Medications
DEA’s Office of Diversion Control receives requests for hearings on a continuous basis to lower a product’s status on the schedule of controlled substances as it relates to the abuse of a product. DEA
DEA’s Laboratory System
DEA’s forensic chemists analyze pharmaceutical preparations submitted from diversion investigators. DEA
Introduction

Explore the rise of patent medicine industry from the late 1800s through to modern day prescription drugs.
Learn More
Learn More
History of Prescription Drugs
Explore the revolution in therapeutics during the past 120 years. Medicines have been discovered to cure disease and to relieve pain.
Learn More
Learn More
The Science of Drugs

Explore the many substances found to have wonderful healing and pain-relieving properties, but also many that could cause harm if used incorrectly.
Learn More
Learn More
The Controlled Distribution System

Explore the various laws and regulations have been passed by the federal and state governments to regulate and control the manufacture, distribution and dispensing of medicines.
Learn More
Learn More
Prescription Fraud

Explore the laws put in place in response to growing abuse and addiction problems with controlled substances.
Learn More
Learn More
Diversion of Chemicals

Explores the clandestine production of drugs, which is dependent on the availability of chemicals necessary to produce the illicit drug activity.
Learn More
Learn More
Pain Management
Explores the critical balance between promoting pain relief and preventing the diversion and abuse of powerful prescription drugs.
Learn More
Learn More
Lost Talent
Discovery Corner